Phosphine

| |||
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Phosphane
| |||
| Other names
Phosphamine
Phosphorus trihydride Phosphorated hydrogen | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.029.328 | ||
| EC Number |
| ||
| 287 | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2199 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| PH3 | |||
| Molar mass | 33.99758 g/mol | ||
| Appearance | Colourless gas | ||
| Odor | fish-like or garlic-like[1] | ||
| Density | 1.379 g/l, gas (25 °C) | ||
| Melting point | −132.8 °C (−207.0 °F; 140.3 K) | ||
| Boiling point | −87.7 °C (−125.9 °F; 185.5 K) | ||
| 31.2 mg/100 ml (17 °C) | |||
| Solubility | Soluble in alcohol, ether, CS2 slightly soluble in benzene, chloroform, ethanol | ||
| Vapor pressure | 41.3 atm (20 °C)[1] | ||
| Conjugate acid | Phosphonium (chemical formula PH+ 4) | ||
Refractive index (nD)
|
2.144 | ||
| Viscosity | 1.1×10−5 Pa⋅s | ||
| Structure | |||
| Trigonal pyramidal | |||
| 0.58 D | |||
| Thermochemistry | |||
Heat capacity (C)
|
37 J/mol⋅K | ||
Std molar
entropy (S |
210 J/mol⋅K[2] | ||
Std enthalpy of
formation (ΔfH⦵298) |
5 kJ/mol[2] | ||
Gibbs free energy (ΔfG˚)
|
13 kJ/mol | ||
| Hazards | |||
| Safety data sheet | ICSC 0694 | ||
| GHS pictograms |    
| ||
| NFPA 704 (fire diamond) | |||
| Flash point | Flammable gas | ||
| 38 °C (100 °F; 311 K) (see text) | |||
| Explosive limits | 1.79–98%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
3.03 mg/kg (rat, oral) | ||
LC50 (median concentration)
|
11 ppm (rat, 4 hr)[3] | ||
LCLo (lowest published)
|
1000 ppm (mammal, 5 min) 270 ppm (mouse, 2 hr) 100 ppm (guinea pig, 4 hr) 50 ppm (cat, 2 hr) 2500 ppm (rabbit, 20 min) 1000 ppm (human, 5 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 0.3 ppm (0.4 mg/m3)[1] | ||
REL (Recommended)
|
TWA 0.3 ppm (0.4 mg/m3), ST 1 ppm (1 mg/m3)[1] | ||
IDLH (Immediate danger)
|
50 ppm[1] | ||
| Related compounds | |||
Other cations
|
|||
Related compounds
|
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Phosphine (IUPAC name: phosphane) is a colourless, flammable, very toxic gas compound with the chemical formula PH3, classed as a pnictogen hydride. Pure phosphine is odourless, but technical grade samples have a highly unpleasant odour like rotting fish, due to the presence of substituted phosphine and diphosphane (P2H4). With traces of P2H4 present, PH3 is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure.
Phosphine is also the general name given to the class of organophosphorus compounds of substituted phosphanes—a class of phosphanes in which the hydrogen atoms have been replaced with organic derivative, having a general formula PR3. Organophosphines are important in catalysts where they complex (adhere) to various metal ions; complexes derived from a chiral phosphine can catalyse reactions to give chiral, enantioenriched products.
History
Philippe Gengembre (1764–1838), a student of Lavoisier, first obtained phosphine in 1783 by heating white phosphorus in an aqueous solution of potash (potassium carbonate).[4][NB 1]
Perhaps because of its strong association with elemental phosphorus, phosphine was once regarded as a gaseous form of the element, but Lavoisier (1789) recognised it as a combination of phosphorus with hydrogen and described it as phosphure d'hydrogène (phosphide of hydrogen).[NB 2]
In 1844, Paul Thénard, son of the French chemist Louis Jacques Thénard, used a cold trap to separate diphosphine from phosphine that had been generated from calcium phosphide, thereby demonstrating that P2H4 is responsible for spontaneous flammability associated with PH3, and also for the characteristic orange/brown color that can form on surfaces, which is a polymerisation product.[5] He considered diphosphine's formula to be PH2, and thus an intermediate between elemental phosphorus, the higher polymers, and phosphine. Calcium phosphide (nominally Ca3P2) produces more P2H4 than other phosphides because of the preponderance of P-P bonds in the starting material.
The name "phosphine" was first used for an organophosphorus compounds in 1857, being analogous to organic amines (NR3).[NB 3][6] The gas PH3 was named "phosphine" by 1865 (or earlier).[7]
Structure and properties
PH3 is a trigonal pyramidal molecule with C3v molecular symmetry. The length of the P−H bond is 1.42 Å, the H−P−H bond angles are 93.5°. The dipole moment is 0.58 D, which increases with substitution of methyl groups in the series: CH3PH2, 1.10 D; (CH3)2PH, 1.23 D; (CH3)3P, 1.19 D. In contrast, the dipole moments of amines decrease with substitution, starting with ammonia, which has a dipole moment of 1.47 D. The low dipole moment and almost orthogonal bond angles lead to the conclusion that in PH3 the P−H bonds are almost entirely pσ(P) – sσ(H) and phosphorus 3s orbital contributes little to the bonding between phosphorus and hydrogen in this molecule. For this reason, the lone pair on phosphorus may be regarded as predominantly formed by the 3s orbital of phosphorus. The upfield chemical shift of the phosphorus atom in the 31P NMR spectrum accords with the conclusion that the lone pair electrons occupy the 3s orbital (Fluck, 1973). This electronic structure leads to a lack of nucleophilicity in general and lack of basicity in particular (pKaH = –14),[8] as well as an ability to form only weak hydrogen bonds.[9]
The aqueous solubility of PH3 is slight; 0.22 cm3 of gas dissolves in 1 cm3 of water. Phosphine dissolves more readily in non-polar solvents than in water because of the non-polar P−H bonds. It is technically amphoteric in water, but acid and base activity is poor. Proton exchange proceeds via a phosphonium (PH+
4) ion in acidic solutions and via phosphanide (PH−
2) at high pH, with equilibrium constants Kb = 4×10−28 and Ka = 41.6×10−29.
Phosphine burns producing a dense white cloud of phosphoric acid:
- PH3 + 2 O2 → H3PO4
Preparation and occurrence
Phosphine may be prepared in a variety of ways.[10] Industrially it can be made by the reaction of white phosphorus with sodium or potassium hydroxide, producing potassium or sodium hypophosphite as a by-product.
- 3 KOH + P4 + 3 H2O → 3 KH2PO2 + PH3
Alternatively, the acid-catalyzed disproportionation of white phosphorus yields phosphoric acid and phosphine. Both routes have industrial significance; the acid route is the preferred method if further reaction of the phosphine to substituted phosphines is needed. The acid route requires purification and pressurizing. It can also be made (as described above) by the hydrolysis of a metal phosphide, such as aluminium phosphide or calcium phosphide. Pure samples of phosphine, free from P2H4, may be prepared using the action of potassium hydroxide on phosphonium iodide (PH4I).
Laboratory routes
It is prepared in the laboratory by disproportionation of phosphorous acid[11]
- 4 H3PO3 → PH3 + 3 H3PO4
Phosphine evolution occurs at around 200 °C. Alternative methods involve the hydrolysis of aluminium phosphide, calcium phosphide, and tris(trimethylsilyl)phosphine.
Occurrence
Phosphine is a constituent of the Earth's atmosphere at very low and highly variable concentrations.[12] It may contribute significantly to the global phosphorus biochemical cycle. The most likely source is reduction of phosphate in decaying organic matter, possibly via partial reductions and disproportionations, since environmental systems do not have known reducing agents of sufficient strength to directly convert phosphate to phosphine.[13]
It is also found in Jupiter's atmosphere.[14]
Possible extraterrestrial biosignature
Phosphine may have been detected in the temperate zone of Venus' atmosphere.[15] It is not expected that phosphine would persist in the Venusian atmosphere, since being subject to ultraviolet radiation, it would eventually be consumed by water and carbon dioxide; thus it would have to be replenished. Phosphine has been proposed as a biosignature for astrobiology. PH3 is associated with anaerobic ecosystems on Earth, which may be indicative of life on anoxic exoplanets. As of 2021[update], no known abiotic process generates phosphine gas on terrestrial planets in appreciable quantities, so detectable amounts of phosphine could indicate life."[16][17][18]
Phosphines
Organophosphines are compounds with the formula PRnH3−n. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Their reactivity is also similar – they can be oxidized to the phosphorus(V) level, they can be protonated and alkylated at phosphorus to give phosphonium salts, and, for primary and secondary derivatives, they can be deprotonated by strong bases to give organophosphide derivatives.
Primary phosphines
Primary phosphines are typically prepared by alkylation of phosphine. Simple alkyl derivatives such as methylphosphine (CH3PH2) are prepared by alkylation of alkali metal derivatives MPH2 (M is Li, Na, or K). Another synthetic route involves treatment of the corresponding chlorophosphines with hydride reagents. For example, reduction of dichlorophenylphosphine with lithium aluminium hydride affords phenylphosphine (PhPH2).
Secondary phosphines
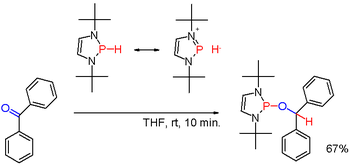
Secondary phosphines are prepared analogously to the primary phosphines. They are also obtained by alkali-metal reductive cleavage of triarylphosphines followed by hydrolysis of the resulting phosphide salt. The latter route is employed to prepare diphenylphosphine (Ph2PH). Diorganophosphinic acids, R2P(O)OH, can also be reduced with diisobutylaluminium hydride. Secondary phosphines are typically protic in character. But when modified with suitable substituents, as in certain (rare) diazaphospholenes (scheme 3), the polarity of the P-H bond can be inverted (see: umpolung) and the resulting phosphine hydride can reduce a carbonyl group as in the example of benzophenone in yet another way.[19]
Tertiary phosphines
Tertiary phosphines are generally obtained by treatment of phosphorus trichloride or triphenylphosphite with organolithium reagents or Grignard reagents. They are commonly used as ligands in coordination chemistry. Tertiary phosphines of the type PRR′R″ are "P-chiral" and optically stable.
Cyclic phosphines
Secondary and tertiary phosphines occur in cyclic forms. Three-membered rings are phosphiranes (unsaturated: phosphirenes), five-membered rings are phospholanes (unsaturated: phosphole), and six-membered rings are phosphinanes.
Applications
Organophosphorus chemistry
Phosphine is a precursor to many organophosphorus compounds. It reacts with formaldehyde in the presence of hydrogen chloride to give tetrakis(hydroxymethyl)phosphonium chloride, which is used in textiles. The hydrophosphination of alkenes is versatile route to a variety of phosphines. For example, in the presence of basic catalysts PH3 adds of Michael acceptors such as acrylonitrile:[20]
- PH3 + 3 CH2=CHZ → P(CH2CH2Z)3 (Z is NO2, CN, or C(O)NH2)
Acid catalysis is applicable to hydrophosphination with isobutylene and related analogues:
- PH3 + R2C=CH2 → R2(CH3)CPH2 (R is Me, alkyl, etc.)
Microelectronics
Phosphine is used as a dopant in the semiconductor industry, and a precursor for the deposition of compound semiconductors. Commercially significant products include gallium phosphide and indium phosphide.[21]
Fumigant
For farm use, pellets of aluminium phosphide, calcium phosphide, or zinc phosphide release phosphine upon contact with atmospheric water or rodents' stomach acid. These pellets also contain agents to reduce the potential for ignition or explosion of the released phosphine. A more recent alternative is the use of phosphine gas itself which requires dilution with either CO2 or N2 or even air to bring it below the flammability point. Use of the gas avoids the issues related with the solid residues left by metal phosphide and results in faster, more efficient control of the target pests.
Because the previously popular fumigant methyl bromide has been phased out in some countries under the Montreal Protocol, phosphine is the only widely used, cost-effective, rapidly acting fumigant that does not leave residues on the stored product. Pests with high levels of resistance toward phosphine have become common in Asia, Australia and Brazil. High level resistance is also likely to occur in other regions, but has not been as closely monitored. Genetic variants that contribute to high level resistance to phosphine have been identified in the dihydrolipoamide dehydrogenase gene.[22] Identification of this gene now allows rapid molecular identification of resistant insects.
Safety
Phosphine gas is denser than air and hence may collect in low-lying areas. It can form explosive mixtures with air, and may also self-ignite.
Phosphine can be absorbed into the body by inhalation. Direct contact with phosphine liquid – although unlikely to occur – may cause frostbite, like other cryogenic liquids. The main target organ of phosphine gas is the respiratory tract.[23] According to the 2009 U.S. National Institute for Occupational Safety and Health (NIOSH) pocket guide, and U.S. Occupational Safety and Health Administration (OSHA) regulation, the 8 hour average respiratory exposure should not exceed 0.3 ppm. NIOSH recommends that the short term respiratory exposure to phosphine gas should not exceed 1 ppm. The Immediately Dangerous to Life or Health level is 50 ppm. Overexposure to phosphine gas causes nausea, vomiting, abdominal pain, diarrhea, thirst, chest tightness, dyspnea (breathing difficulty), muscle pain, chills, stupor or syncope, and pulmonary edema.[24][25] Phosphine has been reported to have the odor of decaying fish or garlic at concentrations below 0.3 ppm. The smell is normally restricted to laboratory areas or phosphine processing since the smell comes from the way the phosphine is extracted from the environment. However, it may occur elsewhere, such as in industrial waste landfills. Exposure to higher concentrations may cause olfactory fatigue.[26]
Toxicity
Deaths have resulted from accidental exposure to fumigation materials containing aluminium phosphide or phosphine.[27][28][29][30] It can be absorbed either by inhalation or transdermally.[27] As a respiratory poison, it affects the transport of oxygen or interferes with the utilization of oxygen by various cells in the body.[29] Exposure results in pulmonary edema (the lungs fill with fluid).[30] Phosphine gas is heavier than air so stays nearer the floor.[31]
Phosphine appears to be mainly a redox toxin, causing cell damage by inducing oxidative stress and mitochondrial dysfunction.[32] Resistance in insects is caused by a mutation in a mitochondrial metabolic gene.[22]
See also
- Diphosphane, H2PPH2, simplified to H4P2
- Diphosphines, R2PPR2, R2P(CH2)nPR2
- Diphosphene, HP=PH
- Diphosphenes, RP=PR′
- Phosphine oxide, R3P=O
- Phosphorane, PR5, R3P=CR2
- Phosphinite, P(OR)R2
- Phosphonite, P(OR)2R
- Phosphite, P(OR)3
- Phosphinate, R2P(RO)O
- Phosphonate, RP(RO)2O
- Phosphate, P(RO)3O
Notes
- ^ For further information about the early history of phosphine, see:
- The Encyclopædia Britannica (1911 edition), vol. 21, p. 480: Phosphorus: Phosphine.
- Thomas Thomson, A System of Chemistry, 6th ed. (London, England: Baldwin, Cradock, and Joy, 1820), vol. 1, p. 272.
- ^ Note:
- On p. 222 of his Traité élémentaire de chimie, vol. 1, (Paris, France: Cuchet, 1789), Lavoisier calls the compound of phosphorus and hydrogen "phosphure d'hydrogène" (hydrogen phosphide). However, on p. 216, he calls the compound of hydrogen and phosphorus "Combinaison inconnue." (unknown combination), yet in a footnote, he says about the reactions of hydrogen with sulfur and with phosphorus: "Ces combinaisons ont lieu dans l'état de gaz & il en résulte du gaz hydrogène sulfurisé & phosphorisé." (These combinations occur in the gaseous state, and there results from them sulfurized and phosphorized hydrogen gas.)
- In Robert Kerr's 1790 English translation of Lavoisier's Traité élémentaire de chimie ... — namely, Lavoisier with Robert Kerr, trans., Elements of Chemistry ... (Edinburgh, Scotland: William Creech, 1790) — Kerr translates Lavoisier's "phosphure d'hydrogène" as "phosphuret of hydrogen" (p. 204), and whereas Lavoisier — on p. 216 of his Traité élémentaire de chimie ... — gave no name to the combination of hydrogen and phosphorus, Kerr calls it "hydruret of phosphorus, or phosphuret of hydrogen" (p. 198). Lavoisier's note about this compound — "Combinaison inconnue." — is translated: "Hitherto unknown." Lavoisier's footnote is translated as: "These combinations take place in the state of gas, and form, respectively, sulphurated and phosphorated oxygen gas." The word "oxygen" in the translation is an error because the original text clearly reads "hydrogène" (hydrogen). (The error was corrected in subsequent editions.)
- ^ In 1857, August Wilhelm von Hofmann announced the synthesis of organic compounds containing phosphorus, which he named "trimethylphosphine" and "triethylphosphine", in analogy with "amine" (organo-nitrogen compounds), "arsine" (organo-arsenic compounds), and "stibine" (organo-antimony compounds).
References
- ^ a b c d e f NIOSH Pocket Guide to Chemical Hazards. "#0505". National Institute for Occupational Safety and Health (NIOSH).
- ^ a b Zumdahl, Steven S. (2009). Chemical Principles (6th ed.). Houghton Mifflin. p. A22. ISBN 978-0-618-94690-7.
- ^ a b "Phosphine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Gengembre (1783) "Mémoire sur un nouveau gas obtenu, par l'action des substances alkalines, sur le phosphore de Kunckel" (Memoir on a new gas obtained by the action of alkaline substances on Kunckel's phosphorus), Mémoires de mathématique et de physique, 10 : 651–658.
- ^ Paul Thénard (1844) "Mémoire sur les combinaisons du phosphore avec l'hydrogène" (Memoir on the compounds of phosphorus with hydrogen), Comptes rendus, 18 : 652–655.
- ^ A.W. Hofmann; Auguste Cahours (1857). "Researches on the phosphorus bases". Proceedings of the Royal Society of London (8): 523–527.
(From page 524:) The bases Me3P and E3P, the products of this reaction, which we propose to call respectively trimethylphosphine and triethylphosphine, ...
- ^ William Odling, A Course of Practical Chemistry Arranged for the Use of Medical Students, 2nd ed. (London, England: Longmans, Green, and Co., 1865), pp. 227, 230.
- ^ Streitwieser, Andrew; Heathcock, Clayton H.; Kosower, Edward M. (2017). Introduction to Organic Chemistry. New Delhi: Medtech (Scientific International, reprint of revised 4th edition, Macmillan, 1998). p. 828. ISBN 9789385998898.
- ^ Sennikov, P. G. (1994). "Weak H-Bonding by Second-Row (PH3, H2S) and Third-Row (AsH3, H2Se) Hydrides". Journal of Physical Chemistry. 98 (19): 4973–4981. doi:10.1021/j100070a006.
- ^ Toy, A. D. F. (1973). The Chemistry of Phosphorus. Oxford, UK: Pergamon Press.
- ^ Gokhale, S. D.; Jolly, W. L., "Phosphine", Inorganic Syntheses 1967, volume 9, pp. 56–58. doi:10.1002/9780470132401.ch17
- ^ Gassmann, G.; van Beusekom, J. E. E.; Glindemann, D. (1996). "Offshore atmospheric phosphine". Naturwissenschaften. 83 (3): 129–131. Bibcode:1996NW.....83..129G. doi:10.1007/BF01142178. S2CID 39778453.
- ^ Roels, J.; Verstraete, W. (2001). "Biological formation of volatile phosphorus compounds, a review paper". Bioresource Technology. 79 (3): 243–250. doi:10.1016/S0960-8524(01)00032-3. PMID 11499578.
- ^ Kaplan, Sarah (11 July 2016). "The first water clouds are found outside our solar system — around a failed star". The Washington Post. Retrieved 14 September 2020.
- ^ Greaves, J.S.; Richards, A.M.S.; Bains, W.; et al. (2020). "Phosphine gas in the cloud decks of Venus". Nature Astronomy. arXiv:2009.06593. Bibcode:2020NatAs.tmp..234G. doi:10.1038/s41550-020-1174-4. S2CID 221655755. Retrieved 14 September 2020.
- ^ Sousa-Silva, Clara; Seager, Sara; Ranjan, Sukrit; Petkowski, Janusz Jurand; Zhan, Zhuchang; Hu, Renyu; Bains, William (11 October 2019). "Phosphine as a Biosignature Gas in Exoplanet Atmospheres". Astrobiology (published February 2020). 20 (2): 235–268. arXiv:1910.05224. Bibcode:2020AsBio..20..235S. doi:10.1089/ast.2018.1954. PMID 31755740. S2CID 204401807.
- ^ Chu, Jennifer (18 December 2019). "A sign that aliens could stink". MIT News.
- ^ "Phosphine Could Signal Existence of Alien Anaerobic Life on Rocky Planets". Sci-News. 26 December 2019.
- ^ Burck, S.; Gudat, D.; Nieger, M.; Du Mont, W.-W. (2006). "P-Hydrogen-Substituted 1,3,2-Diazaphospholenes: Molecular Hydrides". Journal of the American Chemical Society. 128 (12): 3946–3955. doi:10.1021/ja057827j. PMID 16551102.
- ^ Trofimov, Boris A.; Arbuzova, Svetlana N.; Gusarova, Nina K. (1999). "Phosphine in the Synthesis of Organophosphorus Compounds". Russian Chemical Reviews. 68 (3): 215–227. Bibcode:1999RuCRv..68..215T. doi:10.1070/RC1999v068n03ABEH000464.
- ^ Bettermann, G.; Krause, W.; Riess, G.; Hofmann, T. (2002). "Phosphorus Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_527.
- ^ a b Schlipalius, D. I.; Valmas, N.; Tuck, A. G.; Jagadeesan, R.; Ma, L.; Kaur, R.; et al. (2012). "A Core Metabolic Enzyme Mediates Resistance to Phosphine Gas". Science. 338 (6108): 807–810. Bibcode:2012Sci...338..807S. doi:10.1126/science.1224951. PMID 23139334. S2CID 10390339.
- ^ "NIOSH Emergency Response Card". CDC. Retrieved 6 April 2010.
- ^ "NIOSH pocket guide". CDC. 3 February 2009. Retrieved 6 April 2010.
- ^ "WHO – Data Sheets on Pesticides – No. 46: Phosphine". Inchem.org. Archived from the original on 18 February 2010. Retrieved 6 April 2010.
- ^ "NIOSH Alert: Preventing Phosphine Poisoning and Explosions during Fumigation". CDC. 10 July 1995. Retrieved 6 April 2010.
- ^ a b Ido Efrati; Nir Hasson (22 January 2014). "Two toddlers die after Jerusalem home sprayed for pests". Haaretz. Retrieved 23 January 2014.
- ^ "La familia de Alcalá de Guadaíra murió tras inhalar fosfina de unos tapones". RTVE.es (in Spanish). Radio y Televisión Española. EFE. 3 February 2014.
- ^ a b Julia Sisler (13 March 2014). "Deaths of Quebec women in Thailand may have been caused by pesticide". CBC News.
- ^ a b Amy B Wang (3 January 2017). "4 children killed after pesticide released toxic gas underneath their home, police say". Washington Post. Retrieved 6 January 2017.
- ^ "Pesticide blamed in 8-month-old's death in Fort McMurray". CBC News. 23 February 2015. Retrieved 23 February 2015.
- ^ Nath, NS; Bhattacharya, I; Tuck, AG; Schlipalius, DI; Ebert, PR (2011). "Mechanisms of phosphine toxicity". Journal of Toxicology. 2011: 494168. doi:10.1155/2011/494168. PMC 3135219. PMID 21776261.
Further reading
- Fluck, E. (1973). "The Chemistry of Phosphine". Topics in Current Chemistry. Fortschritte der Chemischen Forschung. 35: 1–64. doi:10.1007/BFb0051358. ISBN 3-540-06080-4.
- World Health Organisation (1988). Phosphine and Selected Metal Phosphides. Environmental Health Criteria. 73. Geneva: Joint sponsorship of UNEP, ILO and WHO.
External links
| Wikimedia Commons has media related to Phosphine. |
About the page
Presented content of the Wikipedia article was extracted in 2021-06-13 based on https://en.wikipedia.org/?curid=264583